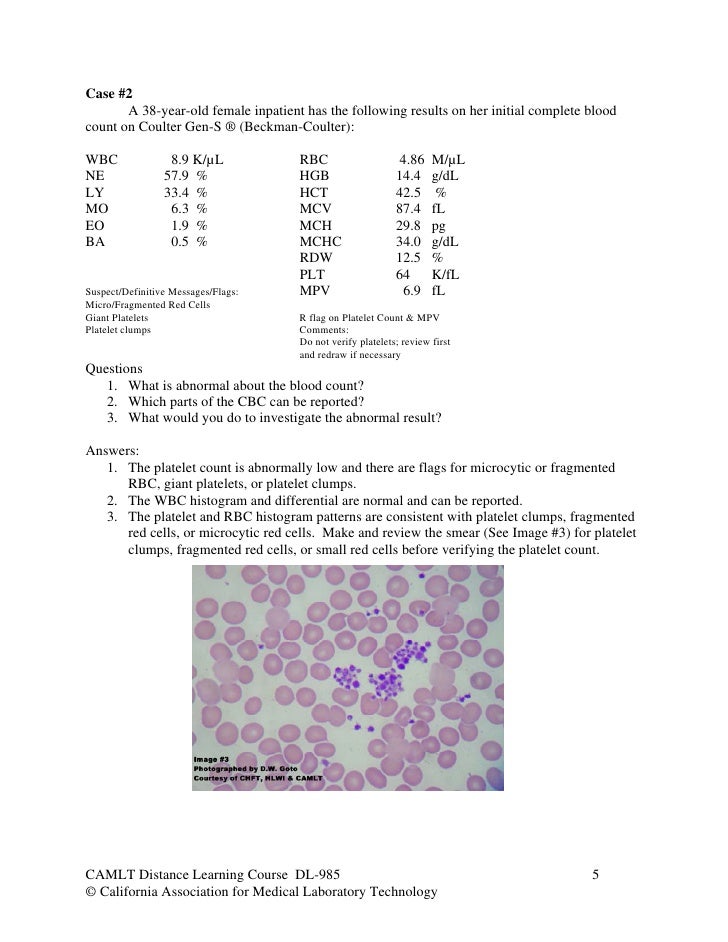
Case Studies In Hematology And Coagulation Pdf Printer

Clinical Laboratory Science (CLSC).
Methods Between July 2001 and April 2004, the Eastern Cooperative Oncology Group (ECOG) conducted a randomized study in which 878 patients with recurrent or advanced non–small-cell lung cancer (stage IIIB or IV) were assigned to chemotherapy with paclitaxel and carboplatin alone (444) or paclitaxel and carboplatin plus bevacizumab (434). Chemotherapy was administered every 3 weeks for six cycles, and bevacizumab was administered every 3 weeks until disease progression was evident or toxic effects were intolerable. Patients with squamous-cell tumors, brain metastases, clinically significant hemoptysis, or inadequate organ function or performance status (ECOG performance status, >1) were excluded. The primary end point was overall survival. Results The median survival was 12.3 months in the group assigned to chemotherapy plus bevacizumab, as compared with 10.3 months in the chemotherapy-alone group (hazard ratio for death, 0. Towerpro 40a Esc Programming there. 79; P=0.003). The median progression-free survival in the two groups was 6.2 and 4.5 months, respectively (hazard ratio for disease progression, 0.66; P. In the United States, lung cancer affects approximately 171,000 people annually and is the leading cause of cancer-related deaths.
Approximately 85% of these patients have non–small-cell lung cancer. The Eastern Cooperative Oncology Group (ECOG) conducted a randomized clinical trial comparing four platin-based, two-drug chemotherapy regimens in more than 1100 patients. The median survival was 8 months, with no significant differences in overall survival among the groups. Although modest progress has been made with the use of chemotherapy in patients with metastatic non–small-cell lung cancer, additional treatment options are needed. Angiogenesis is one of the hallmarks of cancer.